Phase Change Diagram Endothermic Exothermic
11.4: Phase Changes
- Page ID
- 21772
- To summate the free energy changes that accompany phase changes.
Nosotros take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies past perspiration (liquid to gas), and absurd food inside a refrigerator (gas to liquid and vice versa). We use dry out ice, which is solid CO2, equally a refrigerant (solid to gas), and we brand bogus snowfall for skiing and snowboarding by transforming a liquid to a solid. In this section, we examine what happens when whatever of the 3 forms of matter is converted to either of the other 2. These changes of state are often called stage changes. The half-dozen most mutual phase changes are shown in Effigy \(\PageIndex{1}\).
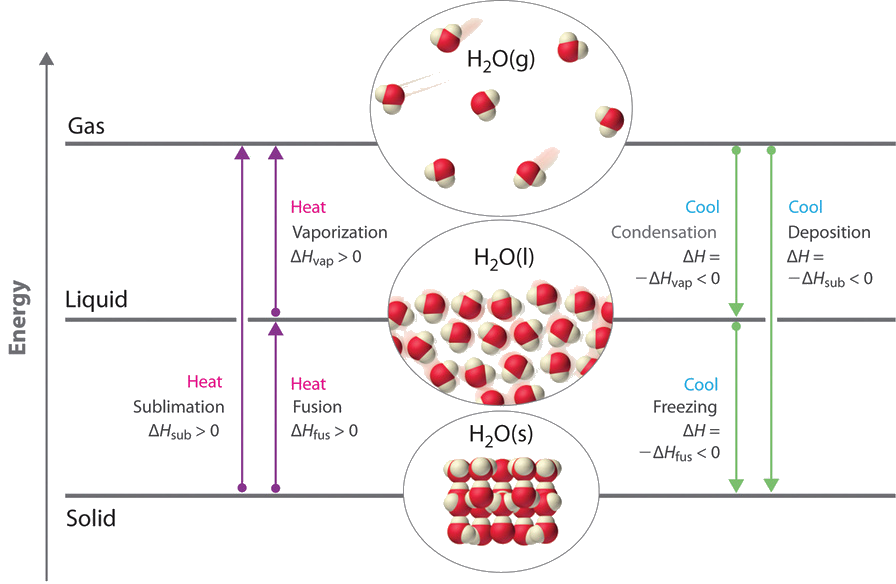
Energy Changes That Back-trail Phase Changes
Phase changes are always accompanied by a alter in the energy of a organization. For instance, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far autonomously, requires an input of energy (heat) to give the molecules enough kinetic energy to let them to overcome the intermolecular bonny forces. The stronger the attractive forces, the more than energy is needed to overcome them. Solids, which are highly ordered, accept the strongest intermolecular interactions, whereas gases, which are very disordered, take the weakest. Thus any transition from a more than ordered to a less ordered state (solid to liquid, liquid to gas, or solid to gas) requires an input of energy; information technology is endothermic. Conversely, whatever transition from a less ordered to a more ordered state (liquid to solid, gas to liquid, or gas to solid) releases energy; it is exothermic. The free energy alter associated with each mutual stage change is shown in Figure \(\PageIndex{1}\).
ΔH is positive for any transition from a more ordered to a less ordered state and negative for a transition from a less ordered to a more ordered country.
Previously, we defined the enthalpy changes associated with various chemical and concrete processes. The melting points and molar enthalpies of fusion (\(ΔH_{fus}\)), the free energy required to convert from a solid to a liquid, a process known as fusion (or melting), as well as the normal boiling points and enthalpies of vaporization (\(ΔH_{vap}\)) of selected compounds are listed in Table \(\PageIndex{1}\).
| Substance | Melting Betoken (°C) | ΔHfus (kJ/mol) | Humid Point (°C) | ΔHvap (kJ/mol) |
|---|---|---|---|---|
| Ntwo | −210.0 | 0.71 | −195.8 | five.six |
| HCl | −114.2 | 2.00 | −85.i | xvi.2 |
| Br2 | −seven.2 | x.vi | 58.8 | xxx.0 |
| CCl4 | −22.6 | 2.56 | 76.eight | 29.viii |
| CHthreeCHii OH (ethanol) | −114.1 | 4.93 | 78.3 | 38.6 |
| CH3(CH2)fourCHiii (n-hexane) | −95.four | xiii.1 | 68.vii | 28.9 |
| HtwoO | 0 | half-dozen.01 | 100 | 40.7 |
| Na | 97.eight | 2.six | 883 | 97.four |
| NaF | 996 | 33.iv | 1704 | 176.one |
The substances with the highest melting points ordinarily have the highest enthalpies of fusion; they tend to be ionic compounds that are held together by very potent electrostatic interactions. Substances with high boiling points are those with strong intermolecular interactions that must be overcome to convert a liquid to a gas, resulting in high enthalpies of vaporization. The enthalpy of vaporization of a given substance is much greater than its enthalpy of fusion considering it takes more energy to completely separate molecules (conversion from a liquid to a gas) than to enable them just to motion past one another freely (conversion from a solid to a liquid).
Less free energy is needed to allow molecules to move past each other than to separate them totally.
The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The amount of free energy required to sublime 1 mol of a pure solid is the enthalpy of sublimation (ΔH sub). Common substances that sublime at standard temperature and pressure (STP; 0°C, 1 atm) include CO2 (dry ice); iodine (Figure \(\PageIndex{2}\)); naphthalene, a substance used to protect woolen article of clothing confronting moths; and 1,4-dichlorobenzene. Every bit shown in Figure \(\PageIndex{1}\), the enthalpy of sublimation of a substance is the sum of its enthalpies of fusion and vaporization provided all values are at the same T; this is an application of Hess'south law.
\[ΔH_{sub} =ΔH_{fus} +ΔH_{vap} \label{Eq1} \]
Fusion, vaporization, and sublimation are endothermic processes; they occur only with the absorption of oestrus. Anyone who has e'er stepped out of a pond pool on a cool, breezy twenty-four hour period has felt the oestrus loss that accompanies the evaporation of water from the pare. Our bodies utilize this aforementioned miracle to maintain a abiding temperature: nosotros perspire continuously, even when at rest, losing most 600 mL of water daily by evaporation from the skin. We too lose about 400 mL of water as water vapor in the air nosotros exhale, which also contributes to cooling. Refrigerators and air-conditioners operate on a similar principle: heat is absorbed from the object or surface area to be cooled and used to vaporize a depression-boiling-point liquid, such equally ammonia or the chlorofluorocarbons (CFCs) and the hydrofluorocarbons (HCFCs). The vapor is and then transported to a different location and compressed, thus releasing and dissipating the heat. Likewise, ice cubes efficiently absurd a drink not because of their depression temperature but because oestrus is required to convert water ice at 0°C to liquid water at 0°C.
Temperature Curves
The processes on the right side of Effigy \(\PageIndex{one}\)—freezing, condensation, and degradation, which are the reverse of fusion, sublimation, and vaporization—are exothermic. Thus heat pumps that utilize refrigerants are essentially air-conditioners running in reverse. Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils inside a house to provide oestrus. The energy changes that occur during phase changes tin can be quantified by using a heating or cooling curve.
Heating Curves
Figure \(\PageIndex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 1000 sample of water. The sample is initially water ice at 1 atm and −23°C; equally heat is added, the temperature of the ice increases linearly with time. The slope of the line depends on both the mass of the ice and the specific heat (C southward) of ice, which is the number of joules required to raise the temperature of 1 grand of water ice by 1°C. As the temperature of the ice increases, the water molecules in the water ice crystal blot more and more energy and vibrate more than vigorously. At the melting bespeak, they accept enough kinetic free energy to overcome attractive forces and movement with respect to ane another. Equally more heat is added, the temperature of the system does non increment further but remains constant at 0°C until all the ice has melted. Once all the ice has been converted to liquid water, the temperature of the water again begins to increase. Now, still, the temperature increases more than slowly than before because the specific heat capacity of water is greater than that of ice. When the temperature of the water reaches 100°C, the water begins to boil. Here, as well, the temperature remains constant at 100°C until all the water has been converted to steam. At this betoken, the temperature again begins to rise, but at a faster rate than seen in the other phases considering the heat capacity of steam is less than that of water ice or water.
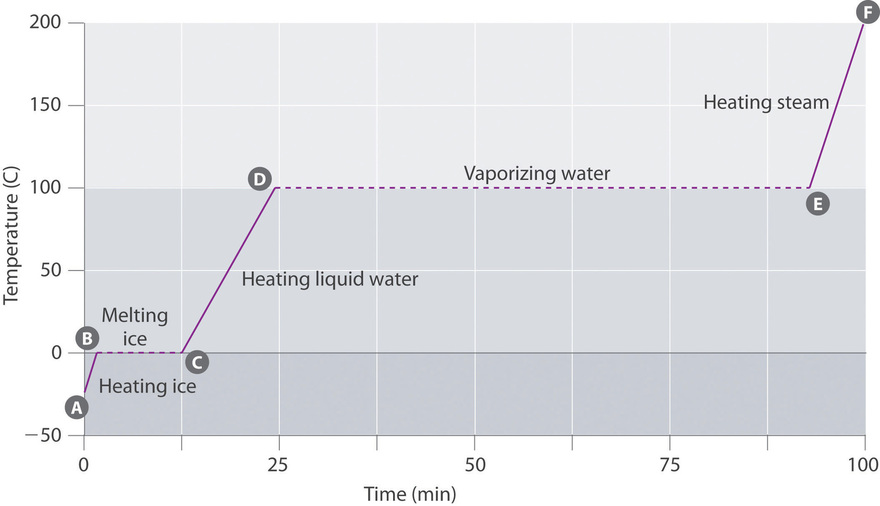
Thus the temperature of a organisation does not change during a stage change. In this example, as long equally even a tiny amount of water ice is present, the temperature of the system remains at 0°C during the melting procedure, and as long every bit even a small amount of liquid water is present, the temperature of the organization remains at 100°C during the humid process. The rate at which heat is added does not touch the temperature of the water ice/water or water/steam mixture because the added rut is being used exclusively to overcome the bonny forces that hold the more than condensed phase together. Many cooks think that food will cook faster if the heat is turned up higher so that the water boils more rapidly. Instead, the pot of water volition boil to dryness sooner, but the temperature of the water does not depend on how vigorously it boils.
The temperature of a sample does not change during a stage change.
If heat is added at a constant rate, as in Figure \(\PageIndex{iii}\), so the length of the horizontal lines, which represents the time during which the temperature does not change, is straight proportional to the magnitude of the enthalpies associated with the phase changes. In Figure \(\PageIndex{iii}\), the horizontal line at 100°C is much longer than the line at 0°C because the enthalpy of vaporization of water is several times greater than the enthalpy of fusion.
A superheated liquid is a sample of a liquid at the temperature and pressure at which information technology should be a gas. Superheated liquids are non stable; the liquid volition eventually eddy, sometimes violently. The miracle of superheating causes "bumping" when a liquid is heated in the laboratory. When a test tube containing water is heated over a Bunsen burner, for example, i portion of the liquid tin can easily become too hot. When the superheated liquid converts to a gas, it can button or "bump" the rest of the liquid out of the test tube. Placing a stirring rod or a small piece of ceramic (a "boiling chip") in the test tube allows bubbling of vapor to form on the surface of the object so the liquid boils instead of becoming superheated. Superheating is the reason a liquid heated in a smooth cup in a microwave oven may not boil until the cup is moved, when the motion of the loving cup allows bubbles to form.
Cooling Curves
The cooling bend, a plot of temperature versus cooling time, in Figure \(\PageIndex{4}\) plots temperature versus time every bit a 75 thousand sample of steam, initially at 1 atm and 200°C, is cooled. Although nosotros might await the cooling curve to be the mirror image of the heating curve in Effigy \(\PageIndex{3}\), the cooling bend is not an identical mirror image. Every bit oestrus is removed from the steam, the temperature falls until information technology reaches 100°C. At this temperature, the steam begins to condense to liquid water. No further temperature alter occurs until all the steam is converted to the liquid; then the temperature again decreases equally the h2o is cooled. We might expect to achieve another plateau at 0°C, where the water is converted to water ice; in reality, yet, this does not always occur. Instead, the temperature ofttimes drops below the freezing point for some fourth dimension, equally shown by the little dip in the cooling curve below 0°C. This region corresponds to an unstable form of the liquid, a supercooled liquid. If the liquid is allowed to stand up, if cooling is continued, or if a small crystal of the solid phase is added (a seed crystal), the supercooled liquid will convert to a solid, sometimes quite suddenly. As the h2o freezes, the temperature increases slightly due to the heat evolved during the freezing procedure and then holds constant at the melting point as the rest of the h2o freezes. Subsequently, the temperature of the ice decreases over again as more estrus is removed from the system.
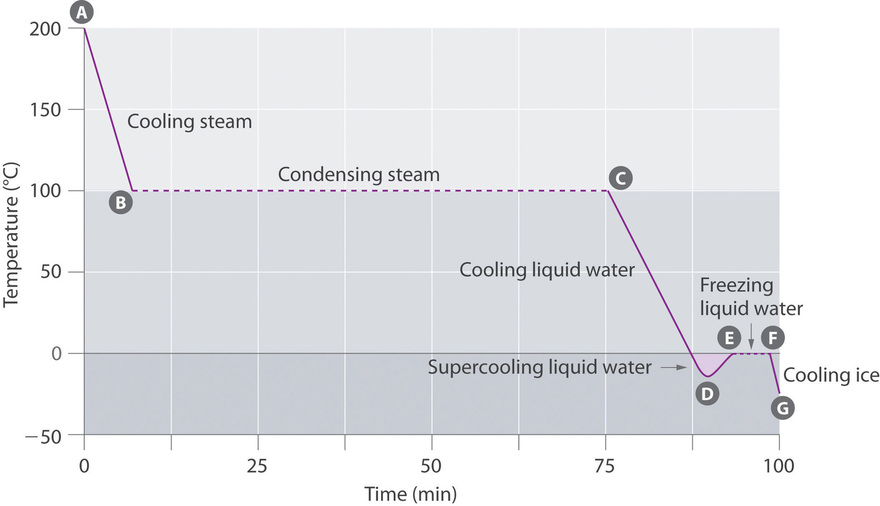
Supercooling effects have a huge impact on Earth's climate. For example, supercooling of water aerosol in clouds tin preclude the clouds from releasing atmospheric precipitation over regions that are persistently arid as a result. Clouds consist of tiny droplets of water, which in principle should be dumbo enough to fall as pelting. In fact, however, the droplets must aggregate to reach a certain size before they tin fall to the footing. Usually a small particle (a nucleus) is required for the droplets to aggregate; the nucleus can be a dust particle, an ice crystal, or a particle of silver iodide dispersed in a deject during seeding (a method of inducing rain). Unfortunately, the small droplets of h2o generally remain every bit a supercooled liquid downward to virtually −10°C, rather than freezing into water ice crystals that are more suitable nuclei for raindrop formation. 1 approach to producing rainfall from an existing cloud is to absurd the water droplets and so that they crystallize to provide nuclei around which raindrops can abound. This is best washed past dispersing small granules of solid COii (dry ice) into the cloud from an airplane. Solid CO2 sublimes straight to the gas at pressures of 1 atm or lower, and the enthalpy of sublimation is substantial (25.3 kJ/mol). As the CO2 sublimes, it absorbs heat from the deject, often with the desired results.
A Video Discussing the Thermodynamics of Phase Changes. Video Source: The Thermodynamics of Phase Changes, YouTube(opens in new window) [youtu.be]
If a 50.0 g ice cube at 0.0°C is added to 500 mL of tea at twenty.0°C, what is the temperature of the tea when the ice cube has just melted? Assume that no heat is transferred to or from the environs. The density of water (and iced tea) is 1.00 g/mL over the range 0°C–20°C, the specific heats of liquid water and ice are iv.184 J/(one thousand•°C) and 2.062 J/(g•°C), respectively, and the enthalpy of fusion of ice is 6.01 kJ/mol.
Given: mass, volume, initial temperature, density, specific heats, and \(ΔH_{fus}\)
Asked for: final temperature
Strategy
Substitute the given values into the general equation relating oestrus gained (by the water ice) to oestrus lost (past the tea) to obtain the last temperature of the mixture.
Solution
When ii substances or objects at unlike temperatures are brought into contact, heat volition flow from the warmer one to the cooler. The corporeality of heat that flows is given by
\[q=mC_sΔT \nonumber \]
where \(q\) is oestrus, \(m\) is mass, \(C_s\) is the specific heat, and \(ΔT\) is the temperature modify. Eventually, the temperatures of the two substances volition become equal at a value somewhere between their initial temperatures. Calculating the temperature of iced tea later calculation an ice cube is slightly more complicated. The general equation relating heat gained and heat lost is still valid, but in this case we also have to take into account the corporeality of estrus required to melt the ice cube from ice at 0.0°C to liquid h2o at 0.0°C.
The amount of heat gained past the ice cube as it melts is adamant by its enthalpy of fusion in kJ/mol:
\[q=nΔH_{fus} \nonumber \]
For our 50.0 one thousand water ice cube:
\[\brainstorm{align*} q_{ice} &= 50.0 chiliad⋅\dfrac{1\: mol}{18.02\:g}⋅6.01\: kJ/mol \\[4pt] &= xvi.7\, kJ \end{align*} \nonumber \]
Thus, when the ice cube has simply melted, it has absorbed xvi.7 kJ of oestrus from the tea. We can and so substitute this value into the first equation to determine the modify in temperature of the tea:
\[q_{tea} = - xvi,700 J = 500 mL⋅\dfrac{1.00\: g}{1\: mL}⋅iv.184 J/(g•°C) ΔT \nonumber \]
\[ΔT = - 7.98 °C = T_f - T_i \nonumber \]
\[T_f = 12.02 °C \nonumber \]
This would be the temperature of the tea when the ice cube has but finished melting; nonetheless, this leaves the melted ice still at 0.0°C. We might more practically want to know what the final temperature of the mixture of tea will be once the melted ice has come to thermal equilibrium with the tea. To determine this, we can add 1 more step to the calculation by plugging in to the general equation relating estrus gained and rut lost once more:
\[\begin{align*} q_{ice} &= - q_{tea} \\[4pt] q_{ice} &= m_{ice}C_sΔT = 50.0g⋅iv.184 J/(g•°C)⋅(T_f - 0.0°C) \\[4pt] &= 209.2 J/°C⋅T_f \end{align*} \nonumber \]
\[q_{tea} = m_{tea}C_sΔT = 500g⋅four.184 J/(g•°C)⋅(T_f - 12.02°C) = 2092 J/°C⋅T_f - 25,150 J \nonumber \]
\[209.2 J/°C⋅T_f = - 2092 J/°C⋅T_f + 25,150 J \nonumber \]
\[2301.2 J/°C⋅T_f = 25,150 J \nonumber \]
\[T_f = x.ix °C \nonumber \]
The terminal temperature is in between the initial temperatures of the tea (12.02 °C) and the melted ice (0.0 °C), so this answer makes sense. In this example, the tea loses much more than rut in melting the ice than in mixing with the cold water, showing the importance of bookkeeping for the heat of phase changes!
Suppose you lot are overtaken by a blizzard while ski touring and you take refuge in a tent. You are thirsty, but you forgot to bring liquid h2o. Y'all have a choice of eating a few handfuls of snow (say 400 g) at −v.0°C immediately to quench your thirst or setting up your propane stove, melting the snowfall, and heating the water to body temperature earlier drinking it. You recall that the survival guide you leafed through at the hotel said something about not eating snow, only you cannot remember why—after all, it's just frozen water. To understand the guide's recommendation, calculate the amount of estrus that your body will have to supply to bring 400 chiliad of snow at −5.0°C to your body'southward internal temperature of 37°C. Use the data in Example \(\PageIndex{i}\)
- Answer
-
200 kJ (4.1 kJ to bring the water ice from −5.0°C to 0.0°C, 133.six kJ to melt the water ice at 0.0°C, and 61.9 kJ to bring the h2o from 0.0°C to 37°C), which is free energy that would not have been expended had y'all beginning melted the snow.
Summary
Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Changes of land are examples of stage changes, or phase transitions. All phase changes are accompanied past changes in the energy of a system. Changes from a more-ordered state to a less-ordered state (such every bit a liquid to a gas) are endothermic. Changes from a less-ordered state to a more-ordered state (such every bit a liquid to a solid) are always exothermic. The conversion of a solid to a liquid is called fusion (or melting). The energy required to cook one mol of a substance is its enthalpy of fusion (ΔH fus). The energy alter required to vaporize 1 mol of a substance is the enthalpy of vaporization (ΔH vap). The direct conversion of a solid to a gas is sublimation. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (ΔH sub) and is the sum of the enthalpies of fusion and vaporization. Plots of the temperature of a substance versus heat added or versus heating fourth dimension at a abiding rate of heating are called heating curves. Heating curves chronicle temperature changes to phase transitions. A superheated liquid, a liquid at a temperature and pressure level at which it should exist a gas, is not stable. A cooling curve is not exactly the contrary of the heating curve because many liquids practice not freeze at the expected temperature. Instead, they form a supercooled liquid, a metastable liquid stage that exists below the normal melting bespeak. Supercooled liquids usually crystallize on standing, or adding a seed crystal of the aforementioned or another substance tin can induce crystallization.
Phase Change Diagram Endothermic Exothermic,
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map:_Chemistry_-_The_Central_Science_%28Brown_et_al.%29/11:_Liquids_and_Intermolecular_Forces/11.04:_Phase_Changes
Posted by: ogdenalling.blogspot.com


0 Response to "Phase Change Diagram Endothermic Exothermic"
Post a Comment